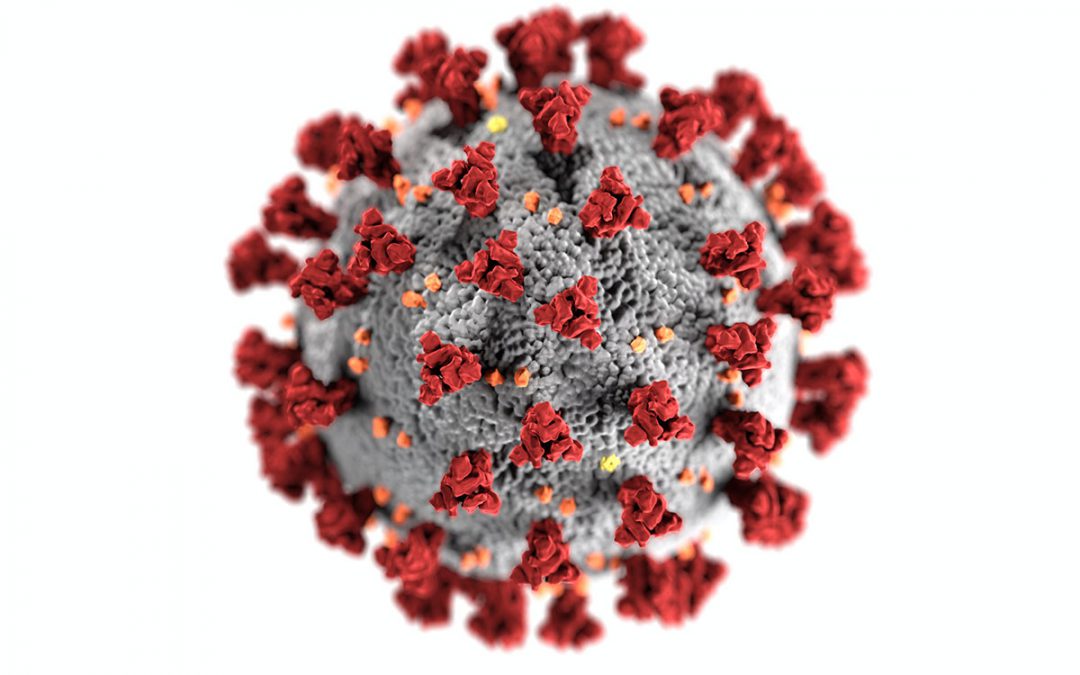
Amidst an unprecedented pandemic, the entire world is in desperate need of treatments for the highly-infectious SARS-CoV-2 virus. When racing against such a rapidly-spreading disease, the speed of drug development is of the utmost importance. An effective therapeutic will heal those already afflicted by COVID-19, allow front-line responders to safely care for patients, and gradually permit the general public to return to their lives. With so much at stake, treatment can’t come soon enough.
Existing approaches to handling new viruses are either too slow to generate new drugs in time or too reliant on existing therapies for related viral diseases. For a disease as unique and fast-moving as this, neither approach is enough.
Monoclonal antibodies (mAb) are a part of the adaptive immune system which tightly bind foreign substances in the body, either directly disabling them, or tagging them for destruction. These proteins have been harnessed by medical research to tackle all kinds of diseases; they have seen success as treatments and preventative measures in many diseases, with several candidates in Phase II clinical trials and even more in development for COVID-19. Antibody drugs, like antibodies produced by the immune system, can be used to mark targets for removal, or to interfere with them directly, which allows for a broad variety of applications. However, standard methods of mAb drug discovery—such as phage display, yeast display, and hybridoma—have slow turnaround times: the time between identifying an antigen target and creating an initial candidate drug is at least 2 months. Even performing the most basic experiments requires a solubilized preparation of the target antigen, the creation of which is an undertaking in and of itself—and universal access is not a guarantee. In the event of an Emerging Infectious Disease crisis, these technologies—which universally rely on time-consuming lab work—are insufficient.
We believe that the best way forward is to do away with discovering drugs in the lab altogether. At Macromoltek, we have developed an entirely computational drug development platform. Instead of developing our drugs in a test tube, we use digital 3D models of antibodies and their targets to predict interactions and effects. These predictions let us select from a large pool of our own unique virtual antibody designs, and generate a small set of candidates. Because we don’t need a physical sample of our target to design drugs, our development process can begin as soon as a protein sequence is released for a target. Drug candidate generation can take as little as a week, and by the time our antibodies make it to the lab, we are already testing potential cures.
Macromoltek’s platform is aiding the ongoing fight against the ongoing COVID-19 pandemic by producing an effective antibody therapeutic in a matter of a few months. Antiviral antibody therapeutics have shown prior success, with several currently being evaluated in clinical trials. Antiviral antibodies that disrupt receptor binding have been proven effective against several targets, such as HIV GP120 glycoprotein, influenza hemagglutinin, and the SARS-CoV spike protein. We have already identified several virtual antibody therapeutic candidates which are undergoing in vitro and in vivo testing over the next few months, before progressing towards clinical trials.
Monica Berrondo, founder of member company Macromoltek, discuss all things antibodies in Episode #2 of ABI’s podcast, ‘Science in the Mall, Y’all’.
Listen Now on your favorite platform:
Acast // SoundCloud // Spotify // iTunes // Google Play // Stitcher